Sulfurous Acid Formula and Properties
Need help with the sulphurous acid formula? Look no further than this comprehensive guide, which breaks down the process into easy-to-follow steps.
Sulfurous Acid – Chemical Formula, Properties, and Uses
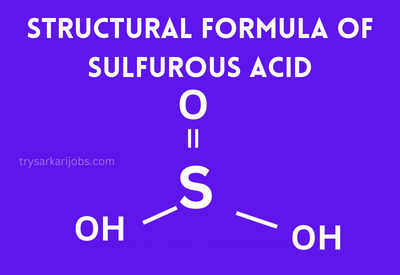
Sulphurous acid is a chemical compound with the formula H2SO3. The molecule is composed of two hydrogen atoms, three oxygen atoms, and one sulfur atom. Sulphurous Acid Nature is Dibasic or Weak Acid, and is an Aqueous Solution of water, & “sulfur dioxide”. It is Colourless & Pungent Odour. Sulphurous Acid is Mainly Used in, Dyes, Drugs, Detergents, Explosives, Various Fertilizers, Pigments, and Inorganic Salts, acids, Petroleum Refinery and Metallurgy Processes.
- The molecular mass of Sulphurous Acid is 82.07 g/mol
- The Chemical Formula for Sulphurous Acid is H2SO3
- The Density of Sulfurous Acid is 1.03 Grams/ Milliliters
- The Boiling Point of Sulfurous Acid is – 60 Degrees Celcius
How DO I Write the Correctly Sulphurous Acid Formula?
F.A.Q For Sulphurous Acid | Sulfurous Acid
What are the Uses of Sulfurous Acid in Daily Life?
What are the Properties of Sulfurous Acid?
What is the Boiling Point of Sulphuric Acid?
What is the Density of Sulphuric Acid?
Read More Articles:
Latest Govt Jobs in Your State
Column Inspection Checklist Pdf
